Aluminium oxide (Al₂O₃) – Definition, Structure, Properties & Industrial Applications
Aluminium oxide, also known as aluminium(III) oxide, is a chemical compound composed of aluminium and oxygen, denoted by the chemical formula Al2O3. Among several aluminium oxides, it is the most prevalent and distinctly recognized as aluminium oxide.
Table of Contents
Aluminium oxide (Al₂O₃) Definition and Significance

Aluminium oxide (Al₂O₃), commonly known as alumina, stands as a fundamental compound with profound importance across a spectrum of industries. This versatile substance, comprising aluminum and oxygen atoms, exhibits remarkable properties that make it indispensable in various applications. Its significance lies not only in its widespread usage but also in its prevalence in nature, serving as the foundation for essential materials vital to our modern way of life.
Prevalence in Nature: Aluminium oxide is one of the most abundant minerals on Earth, constituting a significant portion of the Earth’s crust. It occurs naturally in the form of bauxite, a reddish-brown rock that serves as the primary ore for aluminum production. This natural abundance ensures a steady supply for industrial applications, making it a cornerstone material in numerous sectors.
Role as a Fundamental Compound: Aluminium oxide’s significance transcends its abundance. Its unique combination of properties, including exceptional hardness, thermal stability, and electrical insulating capabilities, positions it as a critical component in diverse industrial processes. From being a catalyst in chemical reactions to enhancing the durability of various materials, aluminium oxide plays a central role in shaping modern technology and innovation.
Aluminium oxide (Al₂O₃) Historical Context
The history of aluminium oxide is intertwined with the evolution of human civilization and scientific discovery. In the early stages of human history, the compound’s existence was not distinctly recognized. However, its presence subtly influenced various cultures as it was found in the vibrant hues of gemstones and the robustness of certain minerals.
Historical Discovery and Early Uses: Aluminium oxide compounds were used in ancient times for their distinctive properties. The compound’s traces were found in the gemstones of antiquity, such as sapphires and rubies, captivating civilizations with their dazzling colors. Ancient cultures, albeit unknowingly, valued aluminium oxide for its aesthetic qualities.
It wasn’t until the 18th century that scientists began to unravel the true nature of aluminium oxide. In 1754, German chemist Andreas Sigismund Marggraf isolated alumina, although he did not recognize it as a distinct compound. Later, in the 19th century, the isolation of aluminum, derived from alumina, marked a significant milestone. The element aluminum, obtained from aluminium oxide, became a precious metal, cherished for its rarity and novel properties.
Evolution as a Vital Material in Modern Applications: The late 19th and early 20th centuries witnessed significant advancements in metallurgy and material science. With the development of efficient extraction methods, aluminium oxide became more accessible, leading to the mass production of aluminum. This transformative period marked the inception of aluminium oxide’s critical role in modern applications.
Over the years, the compound’s applications diversified exponentially. Its high hardness made it indispensable in abrasive materials, contributing to the manufacturing of cutting-edge tools and machinery. In the field of ceramics, its thermal stability became a cornerstone, enabling the production of high-temperature resistant components. Its properties as an excellent insulator found applications in electrical engineering, enhancing the safety and efficiency of electronic devices.
In the realm of catalysis, aluminium oxide emerged as a catalyst support, facilitating numerous chemical processes vital for the production of various goods. Furthermore, its role as a filler in polymers enhanced the mechanical properties of materials used in everyday products, from car components to packaging materials.
Understanding the Structure of Aluminium Oxide
Crystal Structure
Aluminium oxide exhibits a unique crystal structure that significantly influences its properties and applications. In its most stable form, known as alpha alumina, aluminium oxide adopts a hexagonal close-packed (hcp) structure. This arrangement consists of densely packed layers of oxygen atoms, with aluminium ions occupying the octahedral sites between these layers. This specific crystal structure imparts remarkable hardness and thermal stability to aluminium oxide, making it ideal for applications requiring abrasion resistance and high-temperature tolerance.
Additionally, aluminium oxide can exist in other crystalline forms, including the beta and gamma phases. The beta phase has a cubic crystal structure, which, while less common, exhibits distinct properties compared to the alpha phase. The gamma phase, on the other hand, is characterized by a unique, partially disordered structure, giving it specific catalytic properties. These diverse crystalline forms broaden the compound’s utility, allowing it to cater to a wide array of industrial requirements.
Polymorphism
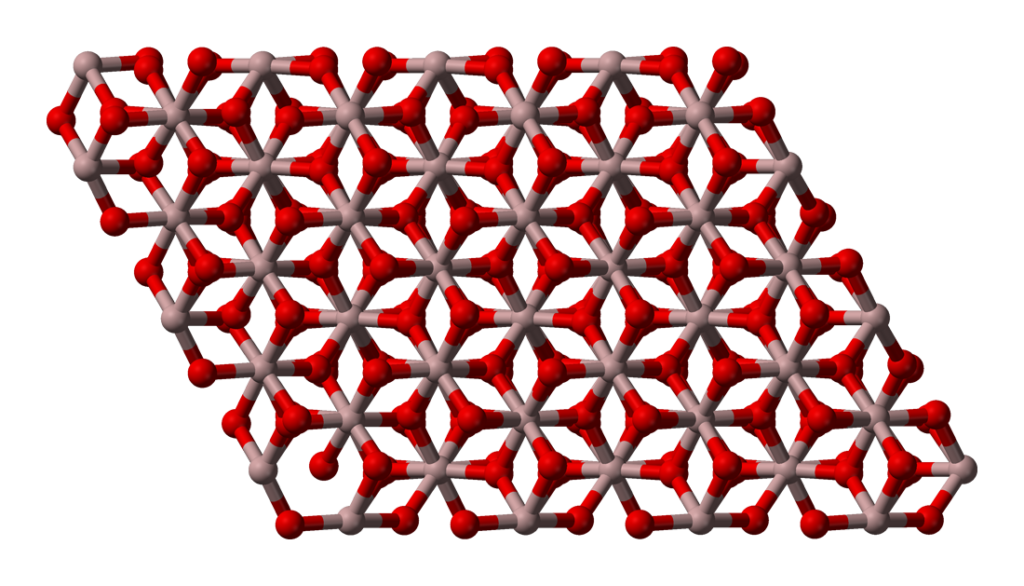
Aluminium oxide’s polymorphic nature refers to its ability to exist in multiple crystal structures without altering its chemical composition. This polymorphism leads to varied behaviors and applications based on the specific form present.
The different polymorphic forms of aluminium oxide profoundly impact its behavior and applications. For instance, alpha alumina, with its tightly packed hexagonal structure, is renowned for its hardness and wear resistance, making it invaluable in abrasive materials and cutting tools. The beta phase, with its cubic arrangement, exhibits unique electrical properties, making it suitable for specialized applications in the field of electronics.
Understanding this polymorphic diversity allows scientists and engineers to tailor aluminium oxide for specific uses, ensuring that its properties align precisely with the requirements of various industries. Whether in the production of ceramics, catalysts, or advanced electronic devices, the polymorphism of aluminium oxide plays a pivotal role in shaping the material’s multifaceted applications.
Unveiling the Properties of Aluminium Oxide
Physical Properties
Aluminium oxide, also known as alumina, possesses distinctive physical properties that render it indispensable in a multitude of applications.
Density: Aluminium oxide exhibits a relatively high density, making it useful in applications where weight and stability are crucial factors. Its dense structure ensures durability and resistance, making it suitable for applications in heavy machinery components and structural materials.
The density of aluminum oxide (Al2O3) is 3.95 g/cm³. However, the density can range from 3.95 to 4.1 g/cm³, depending on the crystal structure.
Here are some other properties of aluminum oxide:
- Molar mass: 101.96 g/mol
- Boiling point: 2,977°C (5,391°F)
- Melting point: 2,072°C (3,762°F)
- Appearance: Solid
- Insoluble in water and most organic solvents
You can measure the density of a thin film of aluminum oxide using x-ray reflectometry.
Melting Point and Boiling Point: With a high melting point of approximately 2,072 degrees Celsius (3,762 degrees Fahrenheit) and a boiling point of about 2,977 degrees Celsius (5,391 degrees Fahrenheit), aluminium oxide showcases exceptional thermal stability. This high-temperature resistance makes it vital in various industries, including the production of refractory materials, crucibles, and components for high-temperature environments such as furnaces and aerospace applications.
Hardness: Aluminium oxide is renowned for its exceptional hardness, ranking 9 on the Mohs scale. This outstanding hardness makes it a key component in the manufacturing of abrasives, grinding wheels, and cutting tools. Its abrasive nature allows for precise machining, shaping, and finishing of materials, making it invaluable in industries ranging from metalworking to gemstone cutting.
The combination of these physical properties contributes significantly to aluminium oxide’s usability in diverse fields. Its density ensures structural stability, while its high melting and boiling points enable applications in extreme heat conditions. Additionally, its hardness provides the necessary abrasive qualities for various manufacturing processes, enhancing efficiency and precision.
Chemical Properties
Reactivity with Acids and Bases: Aluminium oxide exhibits amphoteric behavior, meaning it can react both as an acid and a base. When exposed to acids, it undergoes neutralization reactions, forming salts and water. Conversely, in the presence of bases, it reacts as an acid, forming salts and water as well. This unique property finds applications in chemical processes, where it acts as a buffer and aids in pH regulation.
Stability under Different Conditions: Aluminium oxide is highly stable under a wide range of environmental conditions. It is resistant to corrosion, ensuring its durability even in harsh chemical environments. This stability makes it invaluable in applications where exposure to corrosive substances is prevalent, such as in chemical manufacturing and marine engineering.
Hygroscopicity and Its Implications in Real-World Applications: Aluminium oxide demonstrates hygroscopic behavior, meaning it can absorb and retain moisture from the surrounding atmosphere. This property has implications in real-world applications, particularly in industries where moisture control is vital, such as pharmaceuticals and food processing. Its ability to absorb moisture makes it useful in desiccants and humidity control systems, ensuring the integrity and shelf life of sensitive products.
The Chemical Nature of Aluminium Oxide
Oxidation States
Aluminium oxide (Al₂O₃) reveals the versatile nature of aluminium through its various oxidation states. In aluminium oxide, aluminium typically exists in the +3 oxidation state. In this state, aluminium loses three valence electrons, leaving it with a positive charge of +3. The significance of this oxidation state lies in its stability and reactivity. Aluminium readily forms compounds in the +3 oxidation state, leading to a wide array of chemical interactions.
Significance in Chemical Reactions: The +3 oxidation state of aluminium enables it to participate in redox (reduction-oxidation) reactions. In these reactions, aluminium can either donate or accept electrons, making it a key player in chemical transformations. For instance, in the process of electrolytic reduction, aluminium oxide undergoes a reduction reaction where aluminium gains electrons, producing pure aluminium metal. Conversely, in oxidation reactions, aluminium oxide can lose electrons, leading to the formation of other compounds, such as aluminate ions.
Chemical Reactivity
Acid-Base Reactions Involving Aluminium Oxide: One of the remarkable features of aluminium oxide is its amphoteric nature, meaning it can act as both an acid and a base depending on the circumstances. When reacting with strong acids, aluminium oxide behaves as a basic substance, neutralizing the acid and forming salts and water. Conversely, in the presence of strong bases, aluminium oxide acts as an acidic substance, leading to the formation of salts and water as well. This amphoteric behavior is essential in neutralization reactions, where aluminium oxide can modulate the pH of a solution, making it an invaluable component in various chemical processes and industrial applications.
Reduction Reactions and Its Applications as a Reducing Agent: Aluminium oxide exhibits reactivity in reduction reactions, making it a valuable reducing agent in diverse applications. Its ability to lose oxygen atoms under specific conditions allows it to facilitate reduction processes. For instance, in the reduction of metal ores, aluminium oxide can donate oxygen to other elements, aiding in the extraction of pure metals. This property finds application in the metallurgical industry, where aluminium oxide acts as a reducing agent in the production of metals like iron and titanium.
Moreover, aluminium oxide is employed in the reduction of organic and inorganic compounds. In organic chemistry, it serves as a reducing agent in various synthesis reactions. In industrial settings, it aids in the reduction of pollutants and waste, showcasing its significance in environmental remediation.
From Earth to Industry: Aluminium Oxide in Action

Natural Occurrence
Explore Bauxite Deposits and Their Significance: Bauxite, a sedimentary rock rich in aluminium oxide minerals, stands as the primary source of aluminium oxide in the world. These bauxite deposits are typically found in tropical and subtropical regions, making countries like Australia, Guinea, and Brazil major contributors to the global aluminium oxide supply. Bauxite’s significance lies in its high aluminium content, making it the most economical and efficient raw material for aluminium production.
Discuss the Extraction Processes and Environmental Impact: Bauxite extraction involves strip mining, where topsoil is removed to access the underlying ore. The extracted bauxite undergoes refining processes to extract aluminium oxide. While essential for aluminium production, mining bauxite can have significant environmental impacts, including deforestation, habitat disruption, and water pollution. Responsible mining practices, environmental regulations, and efforts toward sustainable mining techniques are crucial to mitigating these impacts and preserving local ecosystems.
Properties that Define its Versatility
Amphoteric Nature and Its Role as Both an Acid and a Base: One of the defining properties of aluminium oxide is its amphoteric nature. This characteristic enables it to react both as an acid and a base. In acidic conditions, it acts as a base, neutralizing the acid and forming salts and water. Conversely, in basic conditions, it behaves as an acid, leading to the formation of salts and water as well. This unique property makes aluminium oxide invaluable in various chemical processes, where its ability to modulate pH levels is crucial.
Conductivity Properties and Its Applications in Insulating Materials: Aluminium oxide exhibits excellent electrical insulating properties due to its high dielectric strength. This property makes it a preferred choice for insulating materials in the electrical and electronics industries. It is used in the production of insulators, capacitors, and other electronic components where electrical insulation is paramount. Its ability to withstand high voltages and prevent electrical leakage makes it indispensable in ensuring the safety and efficiency of electronic devices.
Production Methods
Bayer Process: Extraction from Bauxite: The Bayer process is the most commonly used method for extracting aluminium oxide from bauxite ore. In this process, bauxite is crushed, digested in a hot solution of sodium hydroxide, and then filtered to remove impurities. The remaining solution, containing dissolved aluminium, is cooled and seeded with aluminium hydroxide crystals. The aluminium hydroxide precipitates out, which is then calcined to produce pure aluminium oxide. The Bayer process provides high yields and is energy-efficient, making it the preferred method for large-scale aluminium oxide production.
Hall-Héroult Process: Obtaining Aluminium Oxide from Alumina: The Hall-Héroult process is employed to obtain aluminium oxide from alumina (aluminium oxide) through electrolysis. In this method, alumina is dissolved in molten cryolite within an electrolytic cell. An electric current is passed through the cell, causing the alumina to dissociate into aluminium and oxygen ions. The aluminium ions migrate to the cathode, where they are reduced and deposited as molten aluminium. Meanwhile, oxygen ions combine to form oxygen gas at the anode. This process allows for the mass production of aluminium and is widely used in the aluminium smelting industry.
Applications Across Industries
Fillers in Polymers
Enhancing Mechanical Properties of Polymers with Aluminium Oxide Fillers: Aluminium oxide finds extensive use as a filler in polymers, reinforcing their mechanical properties. When integrated into polymers, aluminium oxide fillers significantly enhance tensile strength, stiffness, and impact resistance. This reinforcement is vital in industries where lightweight yet robust materials are crucial, such as aerospace and automotive manufacturing.
Glass Industry
Impact on Transparency, Strength, and Durability of Glass Products: In the glass industry, aluminium oxide improves the transparency, strength, and durability of glass products. When added to glass formulations, it enhances optical clarity and increases resistance to breakage. This is particularly valuable in the production of high-quality glassware, lenses, and specialized glass components used in various optical instruments.
Catalytic Applications
Role as a Catalyst Support and Catalyst Component in Various Chemical Processes: Aluminium oxide serves as a catalyst support and component in diverse chemical processes. Its large surface area and porous structure provide an ideal platform for catalytic reactions. It is employed in petrochemical refining, where it acts as a catalyst in processes such as fluid catalytic cracking, facilitating the conversion of crude oil into valuable products like gasoline and diesel.
Gas Purification
Use in Gas Purification Processes to Remove Impurities: Aluminium oxide is crucial in gas purification, where it acts as an absorbent to remove impurities and contaminants from gases. It efficiently adsorbs moisture, acidic gases, and particulate matter, ensuring the purity of gases used in industrial applications, laboratories, and semiconductor manufacturing.
Abrasive Material
Applications in Grinding, Polishing, and Surface Finishing: Due to its exceptional hardness, aluminium oxide is widely used as an abrasive material. It is employed in grinding wheels, sandpaper, and polishing compounds, enabling precise grinding, smoothing, and finishing of various materials, including metals, ceramics, and wood.
Paints and Pigments
Contribution to Color Stability and Durability in Paints and Coatings: In the realm of paints and coatings, aluminium oxide contributes to color stability and durability. It acts as a pigment extender, enhancing the opacity and durability of paints. Additionally, its inert nature ensures that painted surfaces retain their vibrant colors over time, making it valuable in architectural coatings and automotive finishes.
Composite Fiber Production
Strengthening Composite Materials for Various Applications: Aluminium oxide is incorporated into composite materials to enhance their strength and durability. In composite fiber production, it reinforces materials like carbon fiber-reinforced polymers, providing superior mechanical properties. This application is pivotal in industries where lightweight yet strong materials are essential, such as aerospace, sports equipment, and automotive manufacturing.
Armor Manufacturing
Use in Manufacturing Armor Materials Due to Its High Hardness and Strength: Due to its exceptional hardness and strength, aluminium oxide is utilized in the manufacturing of armor materials. In the form of ceramic armor plates, it provides superior ballistic protection for military and law enforcement personnel. Its ability to absorb and disperse the impact of projectiles makes it a critical component in modern body armor systems.
Abrasion Protection
Increasing the Lifespan of Surfaces Through Abrasion Protection: Aluminium oxide coatings are employed to increase the lifespan of surfaces prone to abrasion. These coatings form a protective barrier that resists wear and tear, making them ideal for applications in industries such as manufacturing, where equipment surfaces need to withstand constant friction and abrasion.
Electrical Insulation
Applications in Electrical Insulation Due to High Dielectric Strength: In the electrical industry, aluminium oxide is utilized for electrical insulation purposes due to its high dielectric strength. It is employed in the production of insulators, circuit boards, and electrical components. Its ability to withstand high voltages without conducting electricity ensures the safety and reliability of various electrical devices and systems.
In essence, aluminium oxide’s versatile applications across these diverse industries underscore its importance as a foundational material, driving innovation and enhancing the performance and longevity of various products and technologies.
Exploring Beyond Aluminium Oxide
Aluminium Oxide vs. Other Oxides
Comparative Analysis with Other Oxides, e.g., Silicon Dioxide: Aluminium oxide, while sharing common traits with other oxides like Silicon Dioxide (SiO₂), stands out in specific applications due to its unique properties. Unlike SiO₂, which is well-known for its use in glass manufacturing and electronics, aluminium oxide excels in areas requiring high hardness and wear resistance. It surpasses silicon dioxide in applications demanding superior mechanical strength, making it the preferred choice in abrasive materials, ceramic components, and armor manufacturing.
Specific Applications Where Aluminium Oxide Outshines Other Materials:
- Abrasive Industry: Aluminium oxide’s exceptional hardness makes it a go-to material for abrasives, outperforming many other oxides in grinding, polishing, and sanding applications.
- Ceramic Engineering: In ceramic manufacturing, alumina’s high strength, combined with its resistance to thermal and chemical wear, gives ceramics enhanced durability, especially in high-stress environments like cutting tools and engine components.
- Armor Technology: Aluminium oxide’s remarkable hardness and ability to disperse impact energy make it a standout material in modern body armor, ensuring superior protection against ballistic threats.
Alumina in Ceramics
Use of Alumina in the Ceramic Industry: Alumina, derived from aluminium oxide, is a cornerstone material in the ceramic industry. Its unique combination of properties, including high melting point, exceptional hardness, and excellent thermal and electrical insulation, makes it indispensable in ceramic engineering. Alumina is used to produce a variety of ceramic components, ranging from insulators and spark plugs to cutting tools and grinding media.
Impact on the Strength and Durability of Ceramic Products: The incorporation of alumina significantly enhances the strength and durability of ceramic products. Its high hardness and wear resistance make ceramics more resilient in harsh conditions. In applications like cutting tools, alumina-based ceramics demonstrate exceptional toughness, allowing them to withstand extreme temperatures and mechanical stress. Alumina’s presence ensures that ceramic products can endure challenging environments, making them vital in critical industries such as aerospace, automotive, and electronics.
The versatility and significance of aluminium oxide across diverse industries are truly remarkable. Its unique properties, ranging from exceptional hardness to amphoteric behavior, enable its widespread application in fields as varied as manufacturing, electronics, and defense. Aluminium oxide continues to be a driving force in advancing technology and innovation, shaping the products and technologies we rely on daily.
As we delve deeper into the world of materials science, it is essential to recognize the pivotal role that aluminium oxide plays in shaping our modern world. Its ever-expanding applications, from enhancing the performance of abrasives to ensuring the durability of ceramic products, highlight the material’s enduring importance. We encourage readers to explore further, discovering the endless possibilities offered by this remarkable compound. As technology evolves, aluminium oxide will undoubtedly remain at the forefront of innovation, continuing to inspire scientists, engineers, and inventors worldwide.
FAQs
Aluminum oxide (Al₂O₃) is an ionic compound that contains two aluminum atoms for every three oxygen atoms. It’s also known as alumina, aloxide, aloxite, or alundum.
Aluminum oxide is the most common form of aluminum oxide. It occurs naturally as the mineral corundum, which can form the precious gemstones ruby and sapphire.
Aluminum oxide is used in many industrial, chemical, and commercial applications, including:
-Producing aluminum metal
-As an abrasive
-As a refractory material
-High-temperature electrical and voltage insulators
-Instrumentation parts for thermal test machines
-Seal rings
-Gas laser tubes
-Other laboratory equipment
-Production of ballistic armor
Aluminum oxide is also used as an indirect additive in food substances that are approved by the FDA.
Aluminium oxide is found naturally in the form of bauxite, a reddish-brown rock. Bauxite deposits are abundant in tropical and subtropical regions and serve as the primary source of aluminium oxide.
Aluminium oxide exhibits exceptional hardness, high thermal stability, and amphoteric behavior, meaning it can act as both an acid and a base. These properties make it valuable in industries such as abrasives, ceramics, and electronics.
Aluminium oxide is commonly produced through the Bayer process, where bauxite ore is digested in sodium hydroxide solution to extract alumina. Another method is the Hall-Héroult process, involving the electrolytic reduction of alumina to obtain pure aluminium metal.
Aluminium oxide’s high hardness and abrasive properties make it ideal for grinding, polishing, and surface finishing. Its durability and ability to maintain sharp edges make it a preferred choice in abrasive tools.
Aluminium oxide, also known as alumina (Al₂O₃), is generally considered safe and non-toxic in most applications. It is widely used in various industries, including abrasives, ceramics, electronics, and catalysis. In these contexts, when the material is properly processed and used according to safety guidelines, it poses minimal risk to human health.
However, it’s essential to note that inhaling fine dust particles of aluminium oxide, particularly in occupational settings like manufacturing or machining processes, may pose respiratory health risks. Prolonged exposure to high concentrations of airborne aluminium oxide dust can lead to lung irritation, coughing, and, in extreme cases, conditions like pneumoconiosis (a type of lung disease caused by the inhalation of certain dust particles).
Therefore, it is crucial for workers and individuals handling aluminium oxide in industrial settings to follow recommended safety precautions, including wearing appropriate protective gear such as masks and ensuring proper ventilation to minimize the risk of inhalation.
Aluminium oxide (Al2O3) is used in many applications, including:
Manufacturing aluminium metal, industrial paints, and abrasives
As a fire retardant
As a filler for plastics
As an ingredient in cosmetics and glass
For electric insulation
Making body armor
In refractories, ceramics, polishing, and abrasive applications
In the manufacture of zeolites, coating titania pigments, and as a fire retardant/smoke suppressant
As a substitute for industrial diamond, used for making sandpaper, cutting tools, etc.
In synthetic-sapphire bulletproof windows and ballistics
Aluminium oxide is chemically inert, making it a good filler for bricks, plastics, and heavy clayware. It’s also strong and lightweight, which makes it useful in body armor, vehicle and aircraft armor, and synthetic-sapphire bulletproof windows.
Aluminum oxide (Al2O3) is called aluminum oxide because it’s an ionic compound with the formula Al2O3. This means that it has two aluminum atoms for every three oxygen atoms. Any element that combines with oxygen is an oxide.
The IUPAC name for aluminum oxide is aluminum (III) oxide. It’s the only stable oxide known.
The formula for aluminum oxide is written as Al2O3, which tells us that it has:
Aluminum atoms with +3 charge
Oxygen atoms with -2 charge
In this chemical process, aluminum loses three electrons whereas oxygen gains two electrons.
Aluminum oxide is useful in:
Preventing corrosion
Architectural finishing
Using as a solid desiccant

